- Model Number:Polyisoprene Gloves
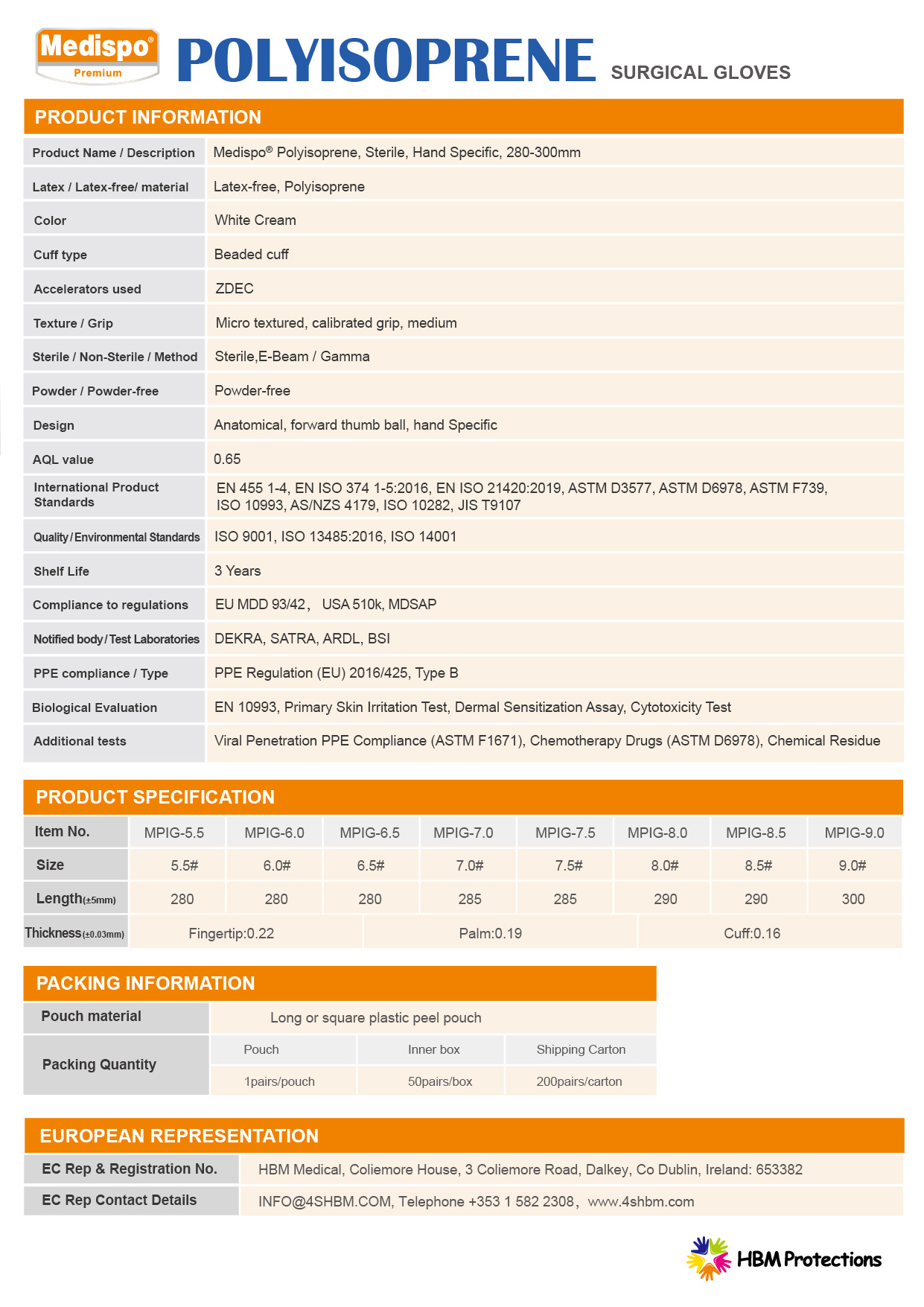
- Latex / Latex-free/ material:Latex-free, Polyisoprene
- Color:White Cream
- Cuff type:Beaded cuff
- Texture / Grip:Micro textured, calibrated grip, medium
- Sterile / Non-Sterile / Method:Sterile,E-Beam / Gamma
- Powder / Powder-free:Powder-free
- Design:Anatomical, forward thumb ball, hand Specific
- Production quality:AQL 0.65/1.5
- International Product Standards:EN 455 1-4, EN ISO 374 1-5:2016, EN ISO 21420:2019, ASTM D3577, ASTM D6978, ASTM F739, ISO 10993, AS/NZS 4179, ISO 10282, JIS T9107
- Quality/ Environmental Standards:ISO 9001, ISO 13485:2016, ISO 14001
- Compliance to regulations:EU MDD 93/42, USA 510k, MDSAP
- Notified body/Test Laboratories:DEKRA, SATRA, ARDL, BSI
- Biological Evaluation:EN 10993, Primary Skin Irritation Test, Dermal Sensitization Assay, Cytotoxicity Test
- Additional tests:Viral Penetration PPE Compliance (ASTM F1671), Chemotherapy Drugs (ASTM D6978), Chemical Residue
- MOQ: 50,000 Pairs
- Size: 6#; 6.5#; 7#; 7.5#; 8.0#; 8.5#; 9#
- Package:1pair/bag, 50pairs/box, 8boxes/CTN
- Carton size: 546*304*424mm
- 20GP Quantity: 200,000 pairs,40HQ quantity: 440,000 pairs
Details
Our powder free surgeons gloves are rigorously tested to meet or exceed international standards to ensure the protection of clinicians. In a study by Akron Rubber Development Laboratory (Ohio, USA), Medispo gloves are demonstrated to be equivalent or better than other popular brands. Produced in our highly automated facility, each glove is automatically stripped, air-leak tested and auto packed in a temperature-controlled, clean environment. Other features include:
Synthetic, latex free, sterile, powder free.
To make them easier to put on, our gloves are infused with H-gel coating.
Our glove performance is equivalent to other popular surgical glove brands–Biogel, Ansell, Medline, Cardinal Health–according to Akron lab report.
Tested safe against permeation by ARDL against 18 chemotherapy drugs under ASTM D6978-05.
Gloves meet or exceed ISO, EN, ATSM standards.
Tested high quality exceeds AQL 0.65.
In compliance with European Medical Device Directive 93/42/EEC Class IIa.