- Model Number:TPC Underglove
- Latex / Latex-free/ material:Natural rubber latex with H-Gel TM coating
- Color:Green
- Cuff type:Beaded cuff
- Texture / Grip:Micro textured, calibrated grip, medium
- Sterile / Non-Sterile / Method:Sterile,E-Beam / Gamma
- Powder / Powder-free:Powder-free
- Design:Anatomical, forward thumb ball, hand Specific
- Production quality:AQL 0.65/1.5
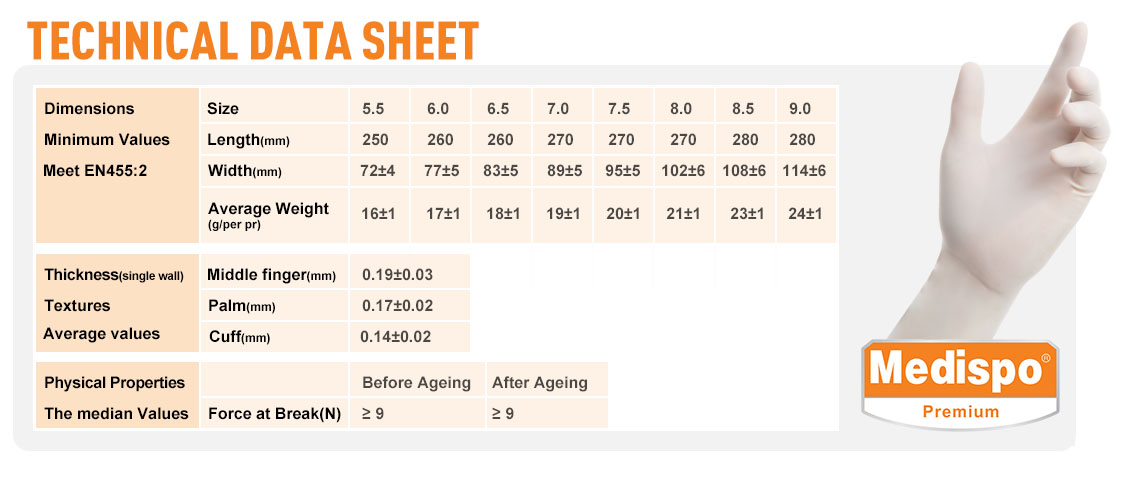
- International Product Standards:EN 455 1-4, EN ISO 374 1-5:2016, EN ISO 21420:2019, ASTM D3577, ASTM D6978, ASTM F739, ISO 10993, AS/NZS 4179, ISO 10282, JIS T9107
- Quality/ Environmental Standards:ISO 9001, ISO 13485:2016, ISO 14001
- Compliance to regulations:EU MDD 93/42, USA 510k, MDSAP
- Notified body/Test Laboratories:DEKRA, SATRA, ARDL, BSI
- Biological Evaluation:EN 10993, Primary Skin Irritation Test, Dermal Sensitization Assay, Cytotoxicity Test
- Additional tests:Viral Penetration PPE Compliance (ASTM F1671), Chemotherapy Drugs (ASTM D6978), Chemical Residue
- MOQ: 50,000 Pairs
- Size: 6#; 6.5#; 7#; 7.5#; 8.0#; 8.5#; 9#
- Package:1pair/bag, 50pairs/box, 8boxes/CTN
- Carton size: 546*304*424mm
- 20GP Quantity: 200,000 pairs,40HQ quantity: 440,000 pairs
Details
Our T-Polyure-coated latex gloves are designed for sensitivity and durability. Each glove is stripped, air-leak tested and auto-packed in a temperature-controlled clean environment. Other features include:
Special T-Polyure coating allows for easier dry and damp hand donning.
Not chlorinated which is kinder for the skin and the environment.
Sterile, powder free to help eliminate powder-related post-operative complications
With reliable grip, proven strength and 100% tested, Medispo gloves protect both clinicians and patients.
Meets or exceeds ASTM surgical glove standards.
Manufactured within the quality management system of ISO 13485: 2003, ISO 9001: 2000, ISO 14001: 2004, FDA-QSR and CE Certification.
In compliance with European Medical Device Directive 93/42/EEC Class IIa.